
We also know that a third dose-a first booster after the primary series-is critical to achieving higher levels of protection from the mRNA vaccines against omicron and the subvariants. We have certainly learned with mRNA vaccines that efficacy, particularly against mild disease or asymptomatic infection, is much lower with omicron and sub-lineages of omicron.

That was during a time when the alpha and beta variants were circulating in the U.S., before delta and omicron. It’s expected to get lower efficacy in that higher risk group. It was lower in older adults over 65-about 80%.
Mrna vaccine spike protein dangers trial#
The clinical trial data on the vaccine’s efficacy looked good-90% overall. This is still a big unknown, and we certainly don’t have any data from the U.S. How is this standing up to omicron subvariants? There is no doubt that the risk is much smaller than the risk of myocarditis from COVID-19 infection. They have to be seen in the context of and in relation to the risk of adverse outcomes from COVID-19. Yes-that’s an important point when we talk about vaccine adverse events. this was seen as a potential adverse event in the clinical trials, which is a much smaller group of vaccine recipients, some FDA advisors thought that there should be a warning should Novavax receive an EUA.Īre the chances of getting myocarditis from a COVID-19 vaccine still substantially lower than getting it from COVID-19? It was only when the vaccine was rolled out into the general population and millions of doses were administered. That side effect was not seen in clinical trials of the mRNA vaccines. It’s somewhat similar to myocarditis or pericarditis, inflammation of the tissue around the heart, that has been observed with the mRNA vaccines. It seems to be more common in younger men-although the numbers are very small.

In the Novavax clinical trial of about 40,000 people, there were six cases of myocarditis, or inflammation of the heart muscle, and one in the placebo group. There can be soreness and redness at the site of injection. Protein-based vaccines tend not to have serious side effects. People will also be familiar with tetanus toxoid vaccines or diphtheria toxoid vaccines as part of a childhood vaccine series. There are a number of widely used vaccines that inject the protein of the virus or the pathogen, like the Human papillomavirus (HPV) or the hepatitis B virus. What other vaccines have been built this way? The protein is made outside of the human body and then injected into us, and that induces the immune response. In some ways, this is an older technology. The Novavax vaccine also has an adjuvant, an immune stimulant to get a better immune response. It a combination of spike proteins that form what are called nanoparticles, which group together. The Novavax vaccine a much older technology for vaccine development where, instead of injecting the genetic recipe, we actually inject the protein.

All those vaccines introduce the genetic recipe for the spike protein into our cells, and then our own cells make the protein to which our immune system responds. We have two messenger RNA (mRNA) vaccines and the Johnson & Johnson adenovirus vector vaccine. This is a different type of vaccine than what we currently have under EUA here in the United States. What makes this vaccine unique from other COVID-19 vaccines in the U.S.?
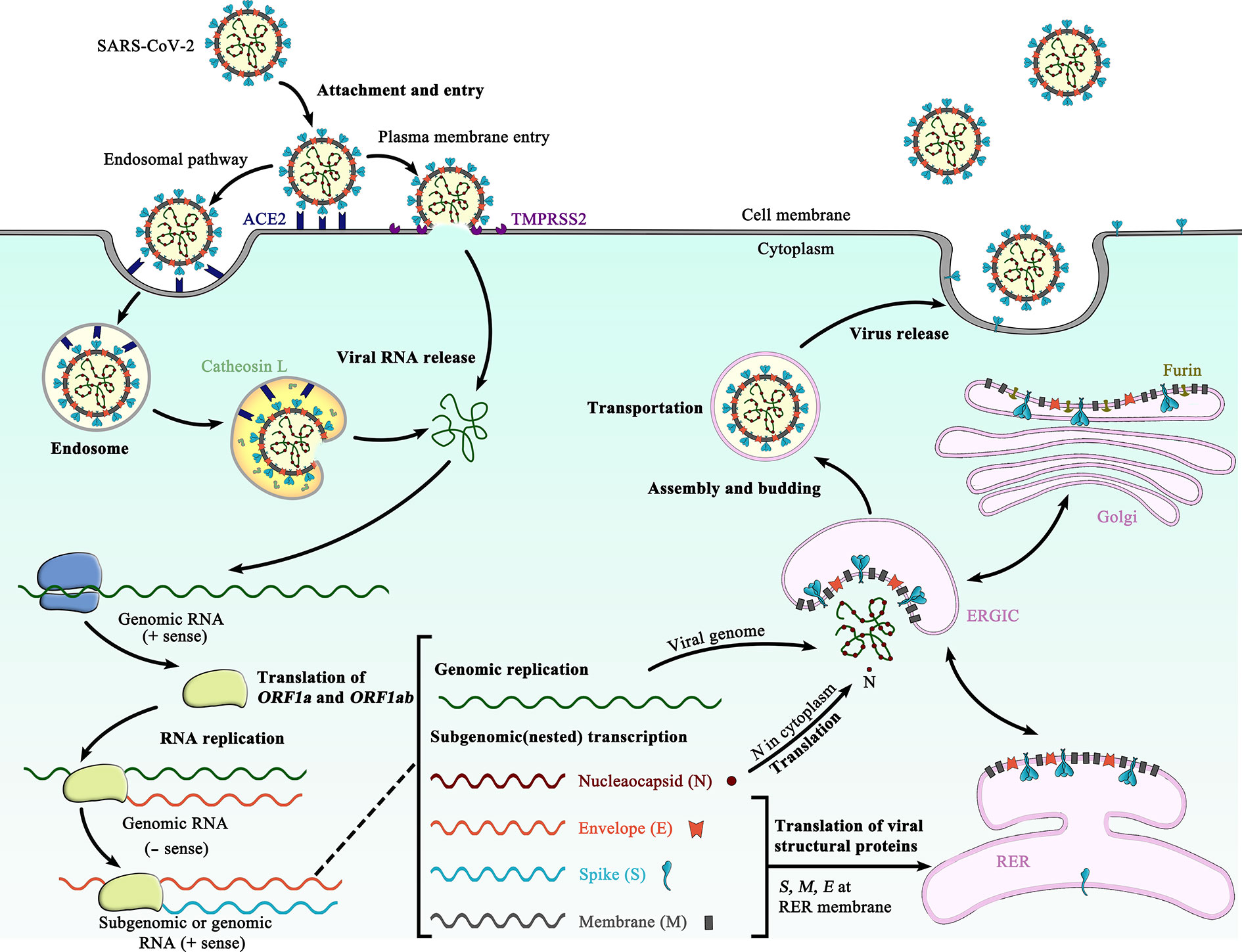
Moss, MD, MPH, executive director of the International Vaccine Access Center, talks about the Novavax vaccine, what’s behind the delay, and what we might expect to see next in the process. The FDA's advisory panel recommended issuing the EUA in June, but authorization was delayed to allow the agency to investigate the company's manufacturing processes for this vaccine. On July 13, FDA granted Emergency Use Authorization (EUA) for the two-dose Novavax COVID-19 vaccine for people 18 and over.


 0 kommentar(er)
0 kommentar(er)
